With a little bit of physical effort and a few simple ingredients, you can make your own delicious ice cream in about 10 minutes. This ice cream is vanilla flavored, but you can experiment with other flavors too!
You will need:
- Ziplock bag – gallon size
- Ziplock bag – quart size
- Ice cubes – enough to fill gallon size ziplock bag about half full
- Milk – ½ cup
- Vanilla extract – ½ teaspoon
- Sugar (granulated) – 2 tablespoons
- Salt – about 8 tablespoons
- Dish towel (optional)
Instructions:
1. Open the quart-sized ziplock and pour milk, sugar, and vanilla extract inside.
2. Close the quart-sized ziplock and set aside.
3. Open the gallon-sized ziplock and put in ice cubes so the bag is about half-full of ice.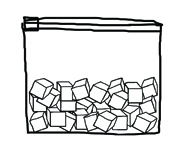
5. Place the closed quart-sized bag inside the gallon-sized bag with ice.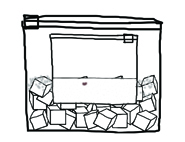
6. Close the gallon-sized bag.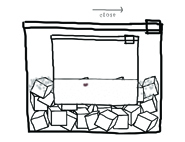
7. Start shaking the bag. You will need to shake for about 5 minutes…and you need to shake it fast….side to side…up and down…you are mixing the ingredients as well as making it cold. Some people shake for 7 or even 10 minutes, but it worked for us when we shook the bag for 5 minutes. You might want to wrap the bag in a dish towel, because it can get cold. You can also shake the bag from the edges to avoid your hands getting too cold.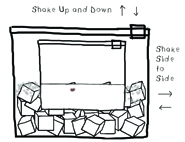
8. Open up the gallon-sized bag and remove the quart-sized bag.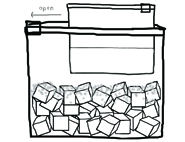
9. Open up the quart-sized bag and enjoy your homemade ice cream!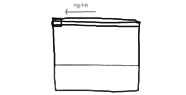
Adding salt to the ice lowers the freezing point and slows the melting rate. Although salt causes the ice to melt, it actually makes it colder. Shaking the contents aerates, (or causes air to circulate throughout) the mixture and gives it a creamy consistency. Using ice and salt is how they made ice cream in Victorian times.
In some parts of the world, salt is put on snow on the road. When ice is at or just below freezing temperatures, it has a wet or slippery surface. However, when ice is well below freezing temperatures, ice has a drier surface and is not very slippery. Therefore, when salt is put on snow on a road, it is to help prevent cars from sliding on a snowy road since salt lowers the freezing point of ice.












Leave a Reply